Abstract
Introduction
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy has been transformative in B-cell lymphoid malignancies. CD30 is a validated target for classical Hodgkin Lymphoma (cHL), as demonstrated by the activity of the anti-CD30 antibody drug conjugate, brentuximab vedotin (BV), making it an attractive target for CAR-T therapy in this disease. In two Phase 1/2 clinical studies, autologous CD30-directed CAR-T-cell (CD30.CAR-T) therapy was well tolerated, with significant clinical activity (72% overall response rate [ORR] and 59% complete response [CR] rate) in relapsed or refractory (R/R) cHL patients after fludarabine-based lymphodepleting (LD) chemotherapy (Ramos et al., 2020). We report here the results of the Pilot segment of a Phase 2 Pivotal trial of autologous CD30.CAR-T in patients with R/R cHL (NCT#04268706).
Methods
This is a Phase 2 single arm, multi-center, multi-national study (U.S. and Europe), enrolling patients (12-75 years) with cHL who have progressed after at least 3 lines of therapy, including chemotherapy, BV, and anti-programmed cell death (PD)-1 antibodies. Previous autologous and/or allogeneic hematopoietic stem cell transplantation (HSCT) is allowed. The study is divided into two parts: a Pilot segment including at minimum 12 patients (for which accrual is complete) and a Pivotal segment planned to enroll approximately 82 patients to obtain at least 67 CD30.CAR-T-treated patients. Eligible patients undergo leukapheresis for manufacture of CD30.CAR-T cells, followed by LD chemotherapy using bendamustine and fludarabine, prior to infusion of CD30.CAR-T with an allowable dose range of 2.0 to 2.7 × 10 8 CD30.CAR-T cells per m 2. Bridging therapy was allowed. Safety is the primary endpoint in the Pilot portion, while ORR as assessed by an Independent Radiology Review Committee per the Lugano criteria (Cheson et al., 2014) is the primary endpoint in the Pivotal segment.
Results
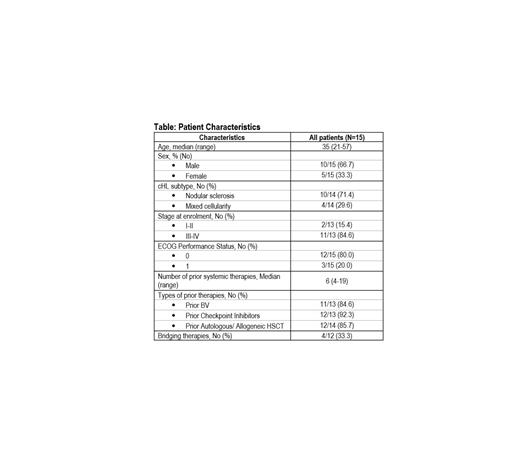
As of 15 July 2021, 17 patients were screened and 15 patients enrolled in the Pilot segment (median age: 35 years [21-57], 66.7% male). The median number of prior therapies was 6, with a range of 4 to 19. Bridging therapy was given in 33.3% of patients. CD30.CAR-T product was successfully manufactured for all patients with a median manufacturing time of 6.3 weeks (5.9-8.0). Of 15 patients enrolled, 14 patients were eligible for treatment with CD30.CAR-T. To date, 12 patients have been treated, with CD30.CAR-T doses ranging from 2.2 to 2.7 × 10 8 cells/m 2. CD30.CAR-T treatment was well tolerated with the most common toxicities reported being hematologic adverse events (AEs)-related to LD chemotherapy. Majority of the AEs were Grade 1-2 anemia, neutropenia and thrombocytopenia. 12.5% of patients had Grade 3 anemia, neutropenia or thrombocytopenia. Other LD-related toxicities include Grade 1-2 nausea, anemia, fatigue, anorexia, gastrointestinal disorder and headache. CD30.CAR-T-related toxicities include Grade 1 cytokine release syndrome and maculopapular rash (reported in the same patient), and Grade 2 ventricular tachycardia in 1 patient. The ORR at Day 42 after CD30.CAR-T infusion, as assessed by investigators, was 100% (5/5). CR and partial response (PR) rates were observed in 4 (80%) and 1 (20%) patients, respectively. Pharmacokinetic analyses of CD30.CAR-T are ongoing.
Conclusion
Preliminary data from the Pilot segment of this study confirms favorable safety profile and excellent anti-tumor responses of CD30.CAR-T in heavily-treated R/R cHL patients. The efficacy and safety of CD30.CAR-T will be further evaluated in the Pivotal segment of this Phase 2 study.
Ahmed: Xencor: Research Funding; Seagen: Research Funding; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding. Flinn: Genentech: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Incyte: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Seagen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Karyopharm Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; BeiGene: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Nurix Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Acerta Pharma: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Takeda: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Iksuda Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Agios: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; AstraZeneca: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Merck: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Verastem: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; MorphoSys: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; AbbVie: Consultancy, Other: All Consultancy and Research Funding payments made to Sarah Cannon Research Institute, Research Funding; Juno Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Trillium Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Infinity Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forma Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Teva: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Gilead Sciences: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Novartis: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Pfizer: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; TG Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; ArQule: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; IGM Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Loxo: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Kite, a Gilead Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Rhizen Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Calithera Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Portola Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Roche: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Curis: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forty Seven: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Celgene: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Unum Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Janssen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Constellation Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Great Point Partners: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Triphase Research & Development Corp.: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Century Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Hutchison MediPharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Vincerx Pharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Sarah Cannon Research Institute: Current Employment; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Seagen: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Unum Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute, Research Funding; Johnson & Johnson: Current holder of individual stocks in a privately-held company; Seattle Genetics: Research Funding. Mei: Epizyme: Research Funding; TG Therapeutics: Research Funding; BMS: Research Funding; GlaxoSmithKline: Honoraria; Morphosys: Honoraria; EUSA: Honoraria; Janssen: Honoraria. Riedell: Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Calibr: Research Funding; Kite/Gilead: Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Research Funding; Tessa Therapeutics: Research Funding; MorphoSys: Research Funding. Armand: Infinity: Consultancy; Pfizer: Consultancy; Kite: Research Funding; Tensha: Research Funding; IGM: Research Funding; Genentech: Consultancy, Research Funding; Roche: Research Funding; Epizyme: Consultancy; AstraZeneca: Consultancy; Regeneron: Consultancy; Enterome: Consultancy; C4: Consultancy; GenMab: Consultancy; Miltenyi: Consultancy; Tessa Therapeutics: Consultancy; Daiichi Sankyo: Consultancy; Morphosys: Consultancy; Celgene: Consultancy; Otsuka: Research Funding; Sigma Tau: Research Funding; ADC Therapeutics: Consultancy; Adaptive: Consultancy, Research Funding; Affimed: Consultancy, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding. Grover: Novartis: Consultancy; ADC: Other: Advisory Board; Genentech: Research Funding; Kite: Other: Advisory Board; Tessa: Consultancy. Engert: Takeda: Consultancy, Honoraria, Research Funding; BMS: Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria; Tessa Therapeutics: Consultancy; Hexal: Honoraria; ADC Therapeutics: Consultancy; Amgen: Honoraria; MSD: Honoraria. Lapteva: Tessa Therapeutics: Consultancy. Nadler: Tessa Therapeutics: Consultancy; Symphogen: Consultancy; Iksuda: Consultancy; Karyopharm: Consultancy. Myo: Tessa Therapeutics: Current Employment. Heslop: Novartis: Membership on an entity's Board of Directors or advisory committees; Kiadis: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kuur Therapeutics: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Marker Therapeutics: Current equity holder in publicly-traded company; Allovir: Current equity holder in publicly-traded company; Gilead: Membership on an entity's Board of Directors or advisory committees; Fresh Wind Biotherapies: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal